Niobium

What is Niobium?
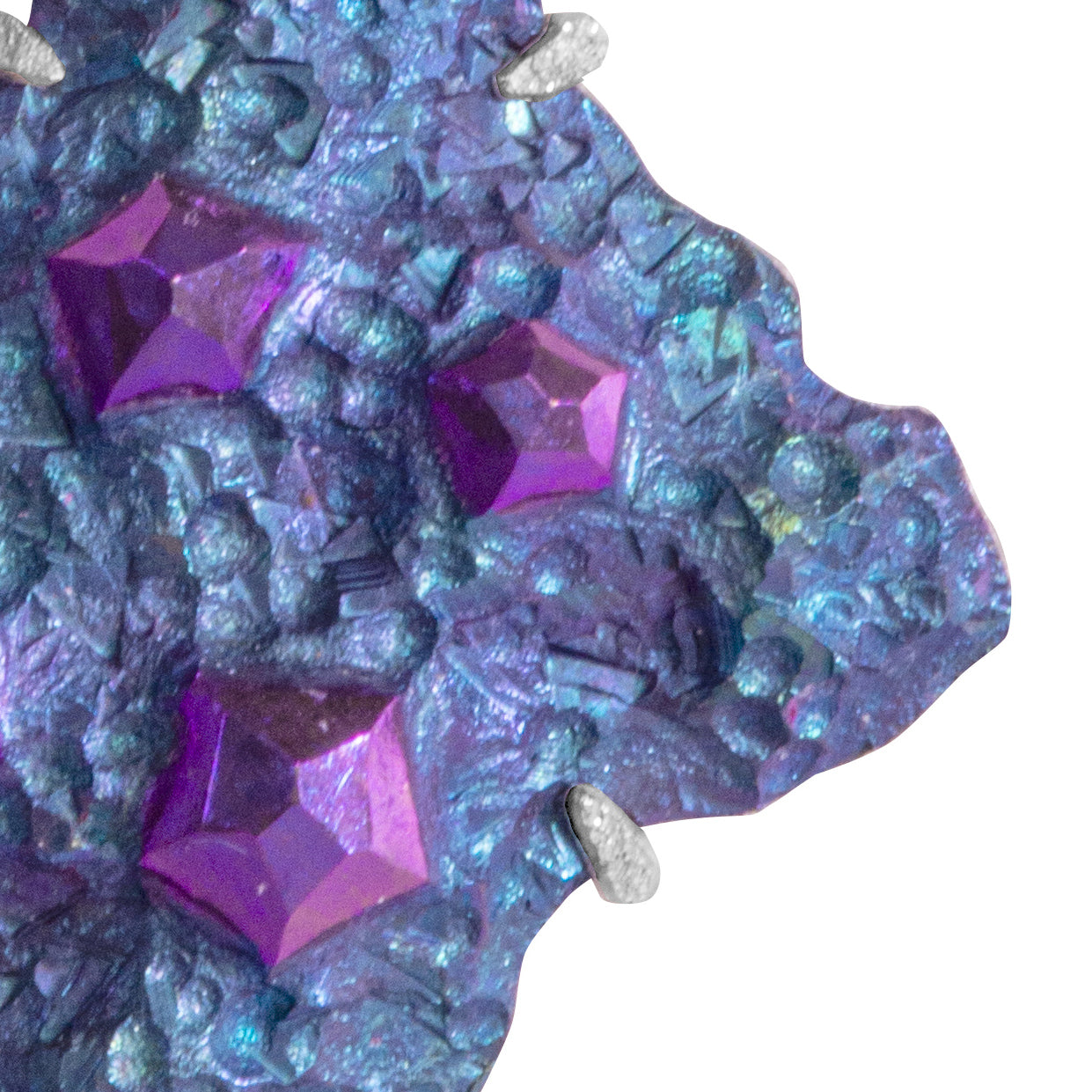
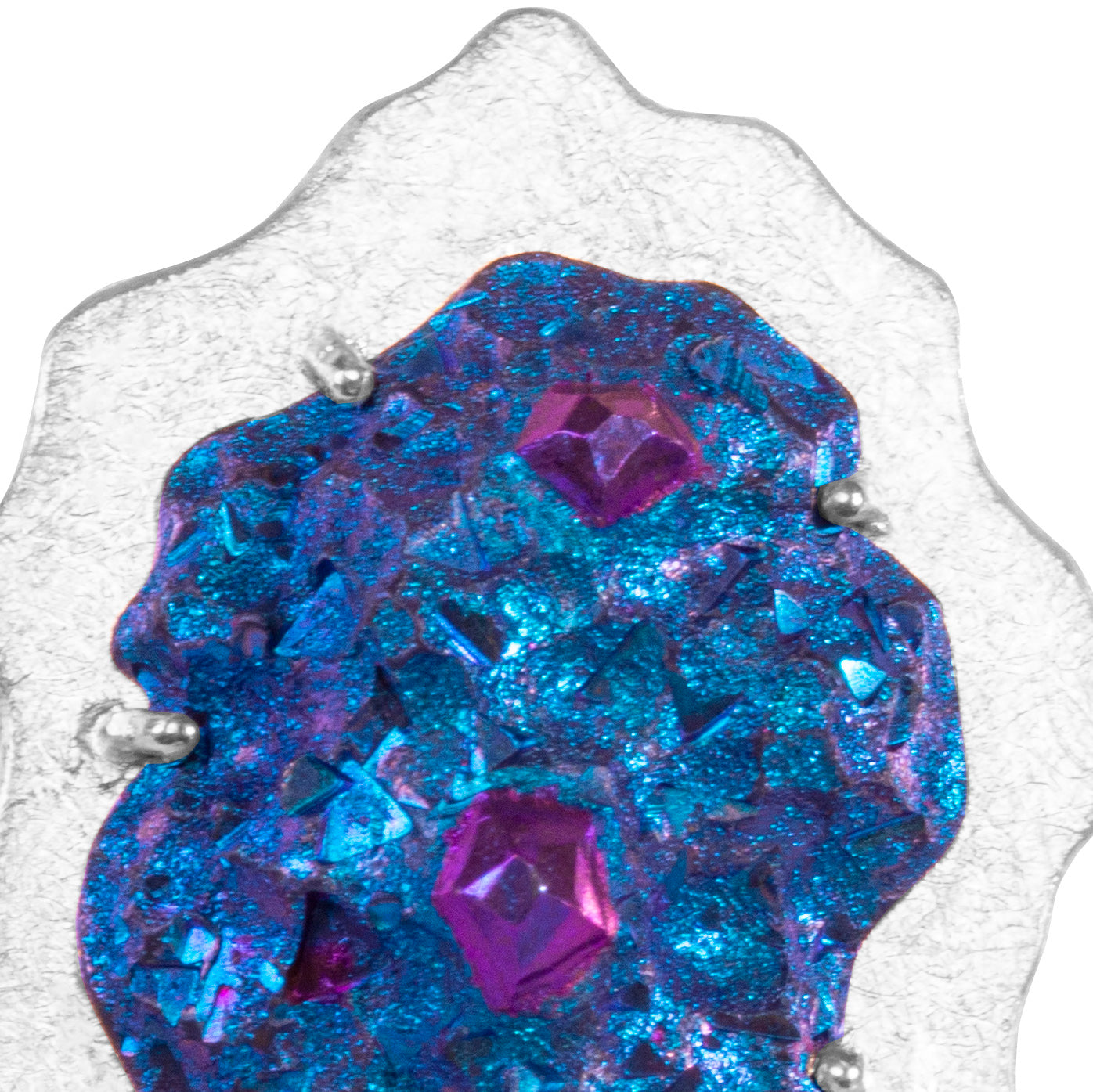
Niobium is a metal in the periodic table (Nb) that’s durable and hypoallergenic. It’s a rare and precious material that’s lightweight and strong — perfect for jewellery!
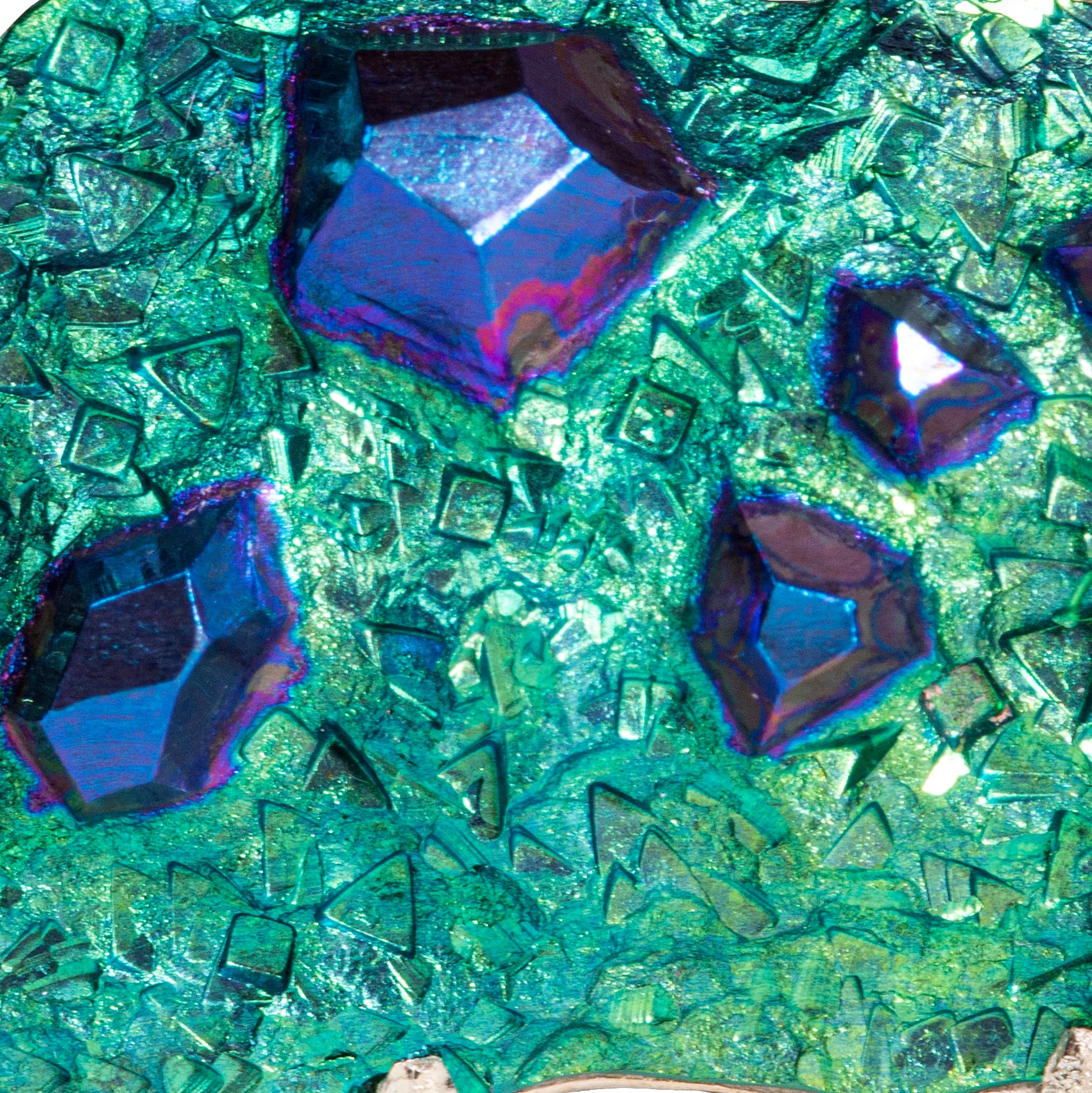
It can be anodised to achieve beautiful colours: yellows, pinks, purples, blues, and greens.
It’s a transition metal and in its pure form can be cold worked from sheet using the technique “chasing and repoussé”.
Voltage Colour Chart
-
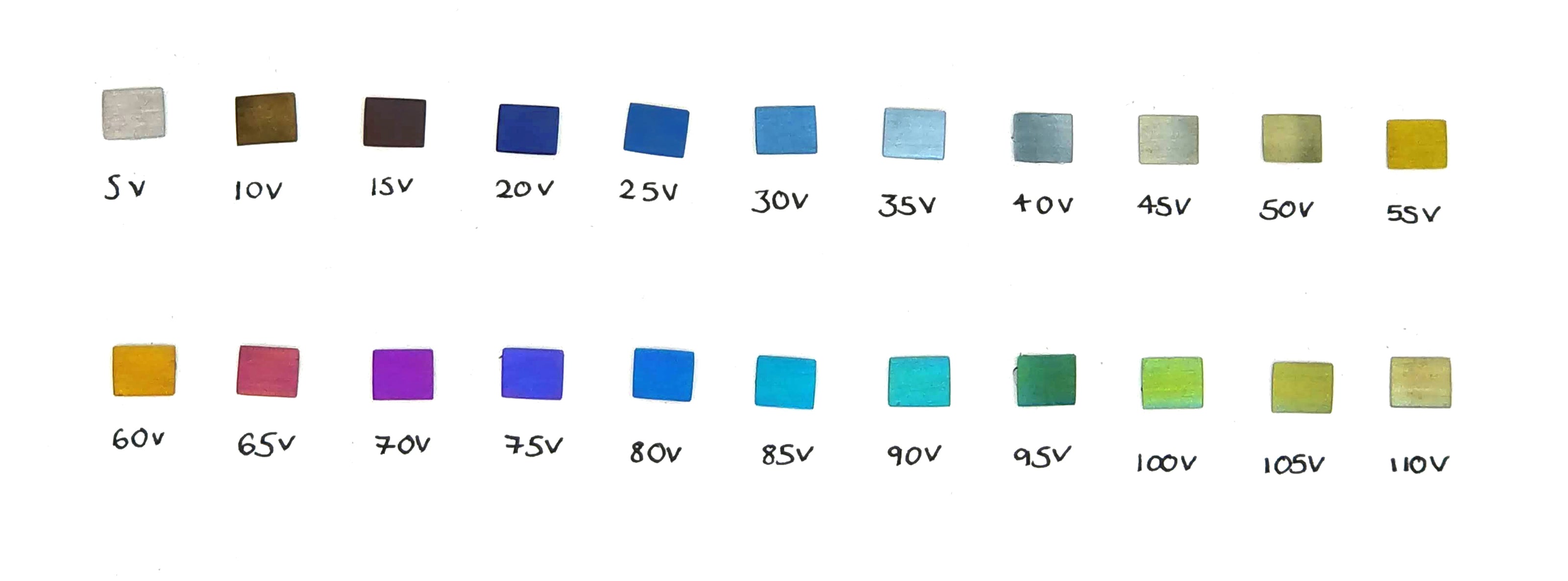
The colour chart above shows the range of colours that niobium can be anodised to at different voltages. Alice's anodising machine can be acurate to 0.1 of a volt, so there are many colours in between the spectrum you see above.
How does anodising work?
-
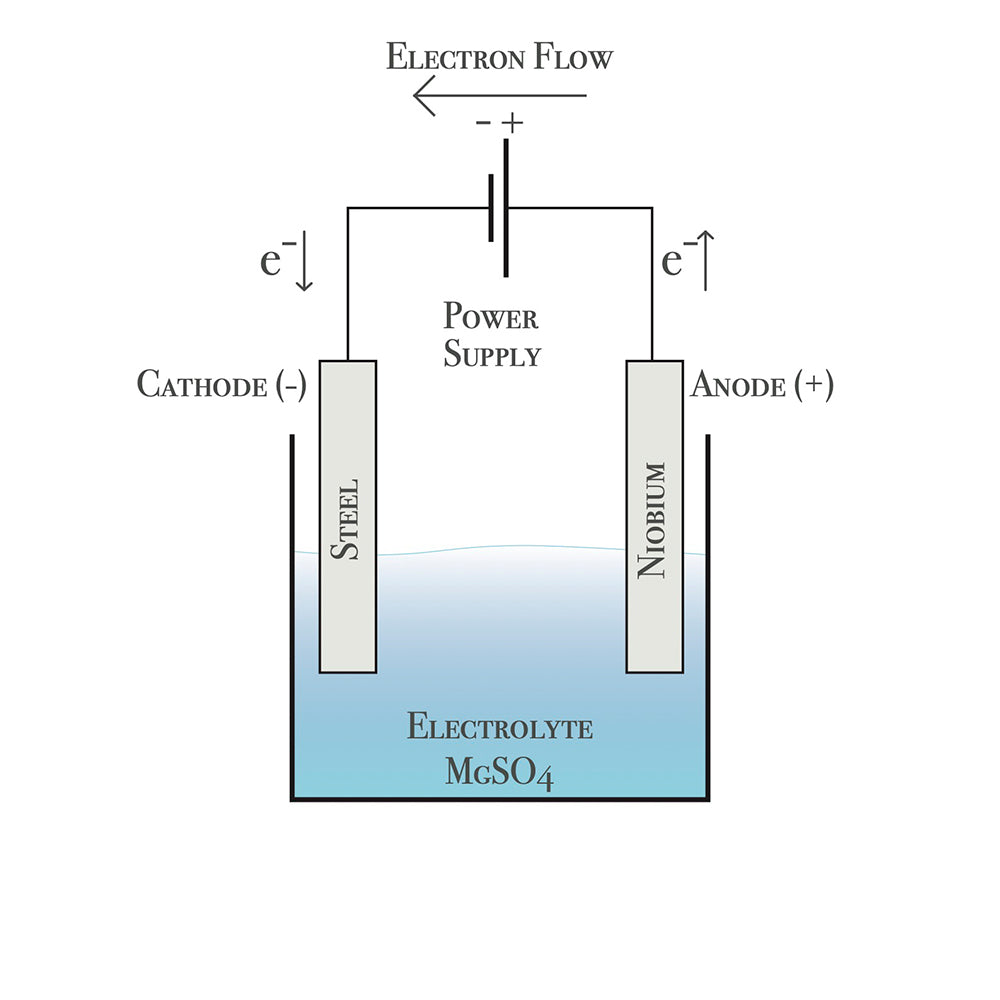
AN ELECTROLYTIC PROCESS
This technique creates a niobium oxide layer on the surface of the niobium metal by passing electricity through the niobium whilst submerged in a bath of bicarbonate of soda dissolved in water.
-

ANODISED BY ALICE
Different voltages create different colours. The higher the voltage, the thicker the oxide layer. Multiple colours can be achieved by masking off areas, or using an electrified brush.
-
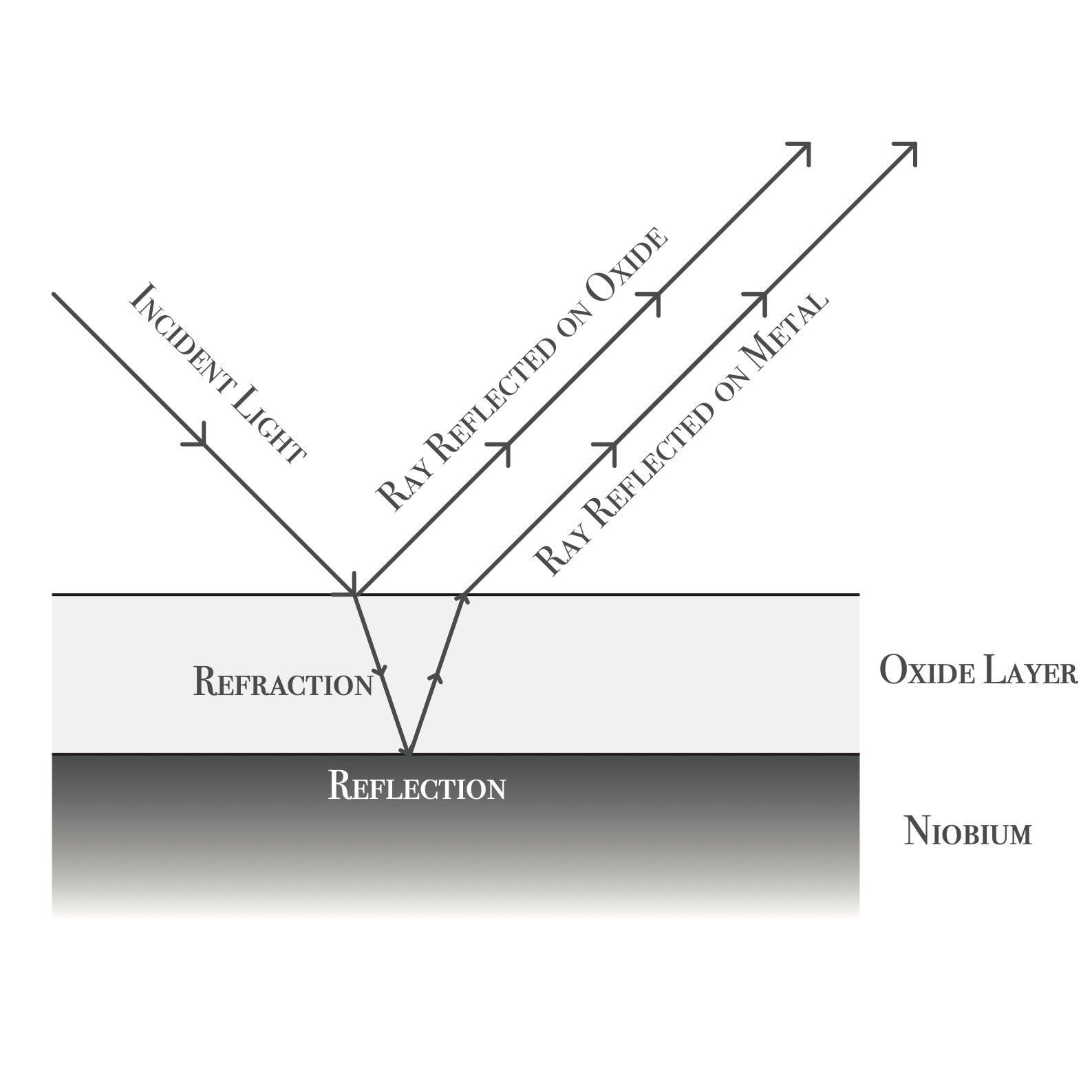
AN OPTICAL ILLUSION
The niobium oxide layer is actually transparent. It’s the way the light refracts and reflects through this material that creates the “interference colours”. Wavelengths of light are cancelled out, leaving the colour that you see.
-

STRUCTURAL COLOURISATION
This works the same way as structural colourisation on butterfly wings. These colours are iridescent and create flashes of different hues at different lights and angles.
Pick your colour!
Go Bespoke
Alice can create a range of colours, shapes and textures in niobium. Each piece is completely unique and can be tailored to you, with your outfits, colours and personality in mind!
Maybe green is your colour? Just contact Alice to start your commissioning journey today.